A majority
of our life is defined by our choices. For the most part, we have the power to
decide everything from what we want to eat today to what we want to spend the
rest of our lives doing. But what about those life-altering decisions that have
already been made for us, sometimes even before we are born? Is it just the
luck of the draw? Should we just accept the cards we were dealt with or is
there something we can do to change the
course of our lives?
For years,
many have succumbed to incurable diseases because the therapies used to treat
the disease were anything but sufficient. People worldwide suffer from
inherited disorders that can cost them later in life and even impact the future
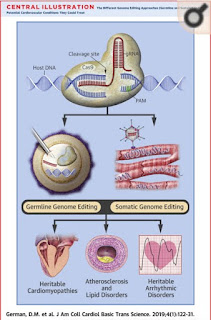
of generations to come. One such disease is hypertrophic cardiomyopathy (HCM).
HCM is a genetic disorder that displays an autosomal dominant inheritance
pattern1. According to the National Human Genome Research Institute,
autosomal dominant explains how certain traits are inherited. Autosomal
signifies that the trait acquired is from a non-sex chromosome; thus, for
humans, autosomal diseases are derived from chromosomes 1 through 22.
Furthermore, dominant explains how many copies of the mutated gene are required
for the disease phenotype. If it is dominant disorder, then only one copy of
the mutated gene will display the disease phenotype. Other examples of
autosomal dominant diseases are Huntington disease and Marfan Syndrome2.
Therefore,
in cases of familial hypertrophic cardiomyopathy, it only takes one mutated
gene to
dictate what the rest of your life will look like. HCM is depicted by the
thickening of the left ventricle of the heart, lowered chamber capacity, and an
enlarged heart1. The muscle walls of heart thicken which restricts
blood flow causing the ventricle to work harder to pump blood to the rest of
the body4. The prevalence of HCM is estimated to occur in around 1
out of 625 people to 1 in 344 people1. The European Society of
Cardiology suggests that a heart wall thickness greater than or equal to 15 mm
is indicative of HCM3. HCM is
a hereditary disorder which affects approximately 0.2% of the population
worldwide1. Common symptoms of HCM include dizziness, fainting,
fatigue, chest pain, shortness of breath, and arrhythmias which can lead to
death7.
While a
number of different mutational events can occur, the most prevalent, accounting
for up to 40% of HCM cases involve mutations in a gene called MYBPC3. This gene is responsible for creating the
cardiac myosin binding protein C, a cardiac protein, and is typically inherited
from at least one parent8. Treatment of patients who have MYBPC3
mutations involve regular monitoring and medications and to open heart surgery
or an implantable cardioverter-defibrillator (ICD)5. While these
treatments options will only alleviate symptoms and are temporary solutions, there
is no cure presently. The survival rate of people with HCM is 98% after 1 year,
94.3% after 3, and 82.2% after 56. While HCM is not necessarily a
death sentence, a person’s mortality is always looming over their shoulder.
People should not have to live in fear, wondering which day will be their last.
Perhaps with genome editing, they won’t have to anymore.
In a 2017
study conducted by Ma et al, the effects of genome editing to correct germline
mutations that cause HCM were explored. Here, healthy gamete donors who had
homozygous and heterozygous heritable MYBPC3 mutations were used. They
used CRISPR-Cas9 to specifically target the heterozygous MYBPC3 mutation in
embryos prior to implantation8. CRISPR-Cas9 is a genome editing
technology that allows for an organism’s DNA to be altered. The technology
involves creation of a small piece of RNA which contains a guide sequence that
attaches to a specific portion of the DNA. That RNA piece will also bind to an
enzyme called Cas9. Essentially, the RNA recognizes the portion of DNA which
needs to be removed from the genome and Cas9 will cut that DNA at the specific
location. After the DNA is cut, a customized DNA sequence will replace the sequence
that was removed from the genome9. Therefore, this technology is especially
promising for individuals with mutations that cause incurable diseases.
Ma et al.
explains that homologous direct repair (HDR) is necessary for gene correction.
They found that the double strand breaks in the human gametes and zygotes were
fixed by using an internal HDR mechanism by using a wild-type allele as a
template. However, in induced pluripotent stem cells, the rate of HDR was much
lower indicating that DNA damage response system behaved differently in gametes
and embryos. One problem, though, is that , there are still off target effects like
non-homologous end joining induced indels that need to be controlled for and
understood8. We are not close to where we need to be, but this is
one step to finding the answer. Undoubtedly, there are many challenges to using
gene therapy. The first of which is limited sample size and the difficulty in
setting clear boundaries10. Furthermore, editing the germline can
cause intended consequences to future generations. Although there is heavy
criticism and controversy surrounding this study, I am hopeful for the future.
Right now, CRISPR-Cas9 has only been tested on animal models and embryos, but
in the future, I expect to see this technology being used in humans who
discover their diseases later in life.
After more testing, the goal is to help those who are victims to genetic
mutations and allow them to live disease free. If this expands to humans and is
successful, individuals will no longer have to succumb to the whimsy of their
genes.
By Rachel Crasta, Master of Medical Sciences Student, University of Kentucky
References:
1.
Marian, Ali J., and
Eugene Braunwald. “Hypertrophic Cardiomyopathy.” Circulation Research, vol. 121,
no. 7, 2017, pp. 749–770., doi:10.1161/circresaha.117.311059.
2.
“Autosomal Dominant.” Genome.gov, NIH, www.genome.gov/genetics-glossary/Autosomal-Dominant.
3.
Authors/Task Force Members, Elliott PM, Anastasakis A,
Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G,
Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG,
Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on
diagnosis and management of hypertrophic cardiomyopathy: the task force for the
diagnosis and management of hypertrophic cardiomyopathy of the european society
of cardiology (ESC). Eur Heart J. 2014;35:2733–2779. doi:
10.1093/eurheartj/ehu284.
4.
“Hypertrophic
Cardiomyopathy.” Www.heart.org,
www.heart.org/en/health-topics/cardiomyopathy/what-is-cardiomyopathy-in-adults/hypertrophic-cardiomyopathy.
5.
Mcnamara, James W., et
al. “MYBPC3 Mutations Are Associated with a Reduced Super-Relaxed State in
Patients with Hypertrophic Cardiomyopathy.” Plos One, vol. 12, no.
6, 2017, doi:10.1371/journal.pone.0180064.
6. Liu,
Qun, et al. “Survival and Prognostic Factors in Hypertrophic Cardiomyopathy: a
Meta-
Analysis.” Nature News, Nature Publishing Group, 20 Sept.
2017,
https://www.nature.com/articles/s41598-017-12289-4.
7.
“Hypertrophic
Cardiomyopathy.” HIE
Multimedia - Hypertrophic Cardiomyopathy, slu.adam.com/content.aspx?productId=117&isArticleLink=false&pid=1&gid=000192.
8.
Ma, Hong, et al.
“Correction of a Pathogenic Gene Mutation in Human Embryos.” Nature, vol. 548, no. 7668,
2017, pp. 413–419.,doi:10.1038/nature23305.
9.
“What Are Genome
Editing and CRISPR-Cas9? - Genetics Home Reference - NIH.” U.S. National Library of Medicine,
National Institutes of Health,
ghr.nlm.nih.gov/primer/genomicresearch/genomeediting.
10. Papasavva, Panayiota, et
al. “Rare Opportunities: CRISPR/Cas-Based Therapy Development for Rare Genetic
Diseases.” Molecular Diagnosis & Therapy, vol. 23, no. 2, 2019,
pp. 201–222., doi:10.1007/s40291-019-00392-3.



Fantastic post Rachel! As CRISPR-Cas9 techniques become more widely understood, I feel the applications for its use will expand exponentially. It is currently estimated that there are over 10,000 heritable diseases in the human genome however, CRISPR's reach into society won't stop there. I believe as society becomes more comfortable with its use, CRISPR will be used more in cosmetic alterations than not. Its use does however raise many ethical dilemmas such as whether it should be allowed in germline alterations or strictly somatic. I feel CRISPR will cause many commotions among our society but will inevitably revolutionize our society as a whole, infiltrating much deeper than the healthcare system.
ReplyDeleteThis was a very interesting topic. A member of an organization I was involved in passed away from this condition without knowing he had it. I am amazed by the results of the CRISPR study you focused on. I think this could be a wonderful solution to diseases similar to the one talked about in your blog post. I hope society becomes more comfortable with exploring our genome so that we can find solutions to currently unavoidable tragedies.
ReplyDeleteGreat post! CRISPR is a fascinating topic which has impressively even gained attention outside of the scientific community for the potential benefits that you mentioned. It's hard not to get excited about the possibilities that genome-editing technologies could create, but as you also mentioned, we are still early in the infant stages of developing these techniques. A big obstacle for CRISPR currently is the potential for undesired pleiotropic effects, suggesting the method is still not optimally precise. Beyond the current scientific limitations of genome-editing, other barriers of development - possibly even greater than the technical aspects - are the ethical concerns that things like CRISPR are already bringing about. These technologies are far more advanced than anything preceding in human history, therefore they inevitably beg certain questions that we have never had to consider. Nevertheless, until these issues are more eminent, it is exciting to watch CRISPR evolve into a promising tool that may soon revolutionize the field of medicine.
ReplyDeleteKomor AC, Badran AH, Liu DR. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell. 2017 Apr 20;169(3):559. doi:10.1016/j.cell.2017.04.005.
CRISPR-Cas9 is an incredible scientific innovation. This is an amazing tool that could prevent the development of so many incurable genetic diseases. I have learned about CRISPR-Cas9 in at least two of my other classes. Even though this is such a great advancement in modern science, it has a lot of controversary surrounding it. Some of the things we discussed in my philosophy class regarding this gene editing technology was that it could potentially cause a larger gap in our societal social classes. This would mainly be due to the fact that the wealthier individuals in our population would have better access because this can be very expensive. CRISPR-Cas9 is not only used to edit gene mutations to prevent diseases, but it could also be used for numerous cosmetic modifications. In this kind of scenario, I can imagine is probably very difficult to define the parameters of when it should or should not be used. However, as time goes on, I think that our society will become more accepting of this advancement because it can improve the qualities of so many lives. I loved this topic and your blog was very fascinating!
ReplyDeleteIf this technology proves to be successful, so many doors would open up. One fear I have regarding this technology is its consistency. Like many medical procedures, there are always risks. What cascade of events could occur with one simple error? Could we induce another, more life-threatening, health issue? The public would be so sensitive to such a mishap that I feel there is minimal room for error if to be used in humans. Regardless, this research is fascinating and successful execution of the CRISPR-Cas9 would be revolutionary.
ReplyDeleteJen Eccleston
Wow, great post. I believe as CRISPR becomes even more notable in society, the possibilities are endless. Genome mapping is becoming more and more normal, I hope to see techniques like this lead to deletion of a genetic irregularity. My only question is how does this genome mapping affect the greater good of genetic diversity.
ReplyDeleteCody R
DeleteThis was a very interesting blog post! The idea of being able to change someone's fate that they had no control over to begin with would be amazing. I'm curious to know how far off this experimentation is from human trials. The biggest issue I see with it is the cost of a procedure like this being done. If it is some astronomical fee with no insurance coverage, it is easy to imagine that the only people this CRISPR technology will serve is the rich. While a small percentage of the population being treated is better than none, it makes me wonder what the future could become. Could it alter genetics so much that the rich develop this "untainted" genetic makeup while the rest of society still has to cope with their genetic misfortune? I fear that could affect much more in life than our overall health, but our social and political hierarchy as well. Of course, this technology is still very far off and the possibilities of helping people are astounding, but unfortunately, with logistics and possible un-regulation, it could amount to much worse.
ReplyDelete-Alivia Larkin
This was a very informative and great blog post, I did not realize how having HCM is just by chance. The post has lots of detail and explains everything very well. It seems that HCM will have an effect on the person health and requires them to be very vigilant with maintaining this after diagnosis. It seems that some treatments for HCA are the sue of beta-blockers or nondihydropyridine calcium-channel blocker and still cause symptoms and disease burden for people with HCM. A study by Heitner et al, examined the use of mavacamten, which is a first class, cardiac specific, small-molecule allosteric modulator of beta-cardiac myosin (1). This drug is intended to innbit the binding of myosin to actin in the heart and reduce the resting and dynamic left ventricular out flow tract (LVOT) in patients with HCM (1). The use of mavacamten is to normalize the function of myosin protein in hypercontractile hearts regardless of the gene mutation in HCM (1). The researchers gave mavacamten to patients for a total of 12 weeks to see if indeed any reversible allosteric modulation of the beta-cardiac myosin will occur in LVOT (1). It was found that mavacamten is well tolerated and did have relief for patient who used this drug in terms of reduction in LVOT (1). Mavacamten was associated with reduction in the LVOT and improvements in exertional capacity was observed. The researchers recommend that this study be done in a larger population and see if indeed mavacamten can be used for treating HCA.
ReplyDelete1. Heitner, S. B., Jacoby, D., Lester, S. J., Owens, A., Wang, A., Zhang, D., ... & Sehnert, A. J. (2019). Mavacamten treatment for obstructive Hypertrophic Cardiomyopathy: a clinical trial. Annals of internal medicine, 170(11), 741-748.
This is great for clinically treating and diagnosing familial hypertrophy cardiomyopathy but what about non-familial cases will crispr be used the same way? It is also important as an ethical question on how we want to treat and diagnose people using crispr technology, as it may be really expensive and a huge burden to families. It is also important to ask patients what other drugs they are on especially in cases if the patient is taking blood thinners, anticoagulants, anti hypertensives and other drugs that target the heart. As of now Crispr may be the only solution to a cure. Great blog post!
ReplyDeleteThis is a great post! I had recently began learning about CRISPR in my biochemistry and my genetics courses and it is a very interesting process. I believe it has incredible applications to the future of healthcare. It is going to be a long process to sort out the limitations and ethical dilemmas involved, but it will one day play a major role. It is a bit controversial right now due to the situation in China in the past few years, however it will become the norm. Great work with this!!
ReplyDeleteI thought this topic was so interesting! Ever since learning about gene editing I have always thought it was such an interesting discovery. Most of the news you hear regarding gene editing is in a very negative light. While some of these fears of the safety and ethics of the technology are extremely legitimate, I also think this technology has so much potential that is overshined. I love hearing about small specific solutions to conditions the human race is impacted by. Researchers are beginning to look for frequently at the role specific genes have on our bodies. In return, this has lead to the discovery of potential genetic changes that could impair conditions like HCM!
ReplyDeleteI didn’t realize that HCM was due to a genetic mutation. I thought it was due to problems with insufficient cardiac output. Regardless, gene therapy is a very interesting novel concept. I have researched the idea of gene therapy in many different types of diseases, one including blindness. Using CRISPR to change the genetic make up of a patient with a disease is a huge stride for curing diseases. I have heard about using viruses to change genetic mutations and wonder if using CRISPR would be a better and safer way to cure patients.
ReplyDeleteI think that this technology is a wonderful way to begin treating untreatable inherited diseases. Whether we approve or not, whether we are ready or not, this is the way the technology is pushing us. I just read an article about a Russian scientist who intends to make more CRISPR-edited babies, in the wake of He Jiankui’s alleged experiment in China. I think that these technologies will be used to greatly improve the lives of many people, but we have to understand that there will be scientists, and perhaps even lay people, who will use it without regard to ethics or safety. I think it is our responsibility as future scientists and care providers to understand as much about this technology as quickly as we can. Since these gene editing tools have become so inexpensive and accessible, we really need to increase our investment in understanding how to make safe, effective treatments in order to get ahead of those who would use these innovations recklessly.
ReplyDeleteCyranoski, David. “Russian Biologist Plans More CRISPR-Edited Babies.” Nature News, Nature Publishing Group, 10 June 2019, https://www.nature.com/articles/d41586-019-01770-x.
Great post, Rachel! I agree with many of the comments that CRISPR can both become a wonderful tool in eliminating genetic disease and a massive detriment to society if the power of gene-editing is abused (I have a linked a very interesting youtube video discussing CRISPR at the bottom of this comment). I am excited to hear that science is exploring new ways to treat such a prevalent disease. I have also recently learned that CRISPR is in trial for combating cancers as well! However, it turns out that using CRISPR/cas9 can also cause cancerous developments in either the treated cells or cells uninvolved in the CRISPR intervention. I believe we have a very long road ahead of us before gene-editing is considered safe enough to be introduced in human trials, however, when that day comes, there will certainly be an inevitable ethical dilemma on our hands regarding the applications of customizing our genome.
ReplyDeletehttps://www.youtube.com/watch?v=jAhjPd4uNFY
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol 36, 765–771 (2018) doi:10.1038/nbt.4192
Great article Rachel! I am amazed by the in-depth research you did on this topic. This topic is another great example of how our genes work. It is interesting, for me at least, to see how in biology we are mainly introduced to how genes work or mutate to our advantage in survival, however they often do the exact opposite too. And despite the discovery of genome-editing, there are a lot of limitations to its effectiveness and moral boundaries. I wonder how the use of genome-editing would change the ethics in the medical field as well or how much of the genome should be edited and what unknown side effects would that treatment end up producing? We can only learn as we go along with trails and experiments, but hopefully the scientists are not taking a blind leap of faith!
ReplyDeleteGenome editing is a crazy concept. But, watching people, especially kids who are too young to understand, go through chronic illnesses and require hospitalizations, treatments, therapies, etc, and struggle through those make me excited to know that there are more therapies/treatments on the horizon. I think it is very interesting that not only can it prevent diseases like Cystic Fibrosis, but can change the myocardium of the heart as you talked about. I'm eager to hear more about the effects of this therapy in the years to come.
ReplyDeletehttps://www.genome.gov/about-genomics/policy-issues/what-is-Genome-Editing
Hey Rachel, I thought that your post was very interesting and very informative. I had never heard of familial hypertrophic cardiomyopathy prior to reading this because it seems that most of the conversation and literature for HCM is related to non-inherited forms. Are the genetic markers for familial HCM related to the gene products that result in non-inherited HCM? I have to wonder, regardless, understanding and treating the genetic basis for the disease is a huge step towards eliminating this issue. I have to wonder at what stage gene therapy can be useful for treating this disease. It is clearly best to correct the mutation in gametes prior to formation of the cardiac tissue, but this is not helpful for people managing the disease as children and adults. At the very least, correcting the mutation could slow or halt the worsening of disease in the myocardium but as we all know crispr/cas9 is not really applicable for human disease so far.
ReplyDeleteThis was a very interesting topic to write about. CRISPR technology is such an amazing concept, but of course, with that, also comes the ethical issues involved. To me, the biggest issue I can see with CRISPR as it is used on human patients is the cost. Especially because HCM is not technically a death sentence, I don't see insurances forking over any money to pay for the procedures involved in gene therapy. Because I'd imagine that this type of therapy would be expensive, you have to consider the population that would be receiving treatment: the wealthy. Not to say they don't deserve the treatment, I just worry about what the far future would look like by serving gene therapy to only the wealthy because they can afford it and creating a superior generation. Of course this is all hypotheticals and I don't necessarily believe that this should be the only thing hindering the furthering of gene therapy into human patients. It would certainly change healthcare drastically and allow many people to live a much fuller life, if it cracks up to all its hoped to be.
ReplyDelete-Alivia Larkin